h1n1 2022 ingredients
Influenza A H1N1 2009 Monovalent Vaccine is a sterile suspension for intramuscular injection. Before the H1N1 pandemic in 2009 the influenza AH1N1 virus had never been identified as a cause of infections in people.

Pandemic Influenza Vaccine Manufacturing Process And Timeline
A single 05 mL dose of Influenza A H1N1 2009 Monovalent Vaccine contains sodium chloride 41 mg monobasic sodium.

. Access More Information On How FluMist Quadrivalent Works At The Official HCP Website. 2021-2022 influenza season contain the following. AFLURIA QUADRIVALENT influenza a virus avictoria25702019 ivr-215 h1n1 antigen propiolactone inactivated influenza a virus acambodiae08263602020 ivr.
May 2017 While health officials recommend an ever increasing number of vaccines for babies and young. Access More Information On How FluMist Quadrivalent Works At The Official HCP Website. 12 - 16 oz.
A BPhuket30732013 BYamagata lineage-like virus. Fluzone intradermal is administered intradermally via a single-dose prefilled microinjection syringe. The H1N1 vaccine Arepanrix has been developed by GlaxoSmithKline using the egg-based production method.
WHO convenes technical consultations. Fluzone Quadrivalent is standardized according to United States Public Health Service requirements and is formulated to contain HA of each of the following four influenza. What ingredients were in the H1N1 vaccine.
Learn More About The Mechanism Of Action. See DESCRIPTION 11 for the complete list of ingredients Influenza. The composition of trivalent influenza vaccines is recommended to include the H1N1 H3N2 and the B Victoria lineage virus.
This means the vaccine viruses are grown in eggs. HHS Purchases Additional H1N1 Vaccine Ingredients. HHS Secretary Kathleen Sebelius announced today that the department will commit 884.
Ad Influenza Vaccine Live Intranasal. List of the 10 NDC products with the active ingredient Influenza A Virus Atasmania5032020 Ivr-221 h3n2 Antigen formaldehyde Inactivated. H1N1pdm09-like or cell culture -propagated AHawaii702019.
Learn More About The Mechanism Of Action. This is true for. The preferred site for administration is over the deltoid muscle.
This year there is a new A H1N1-like virus strain and a new A H3N2-like virus strain when compared to the composition of quadrivalent vaccines for Australia in 2020. The recommendation is for up to 7 days. Components of COVID-19 vaccines authorized by Health Canada.
Weir Keith H1N1 - Pandemic Vaccine Ingredients Over Rated Compared to Homeopathic Properties of Silver H1N1 - Pandemic Vaccine Ingredients Over Rated Compared to. Genetic analyses of this virus have shown that it originated from. FLUAD QUADRIVALENT is standardized according to United States Public Health Service requirements and each 05 mL dose is formulated to contain 15 mcg of hemagglutinin.
For cell-based and recombinant vaccines the component changed from an AHawaii702019 H1N1pdm09-like virus to an AWisconsin5882019 H1N1pdm09-like. The phase IV clinical study is created by eHealthMe based on reports from the FDA and is updated regularly. The clinical trials have shown that BETADINE COLD DEFENSE has a high tolerability no nasal irritation smell or taste828388.
2022 northern hemisphere influenza season. No report of H1n1 influenza is found in people who take Fermented genistein. The committee recommended that the quadrivalent formulation of egg-based influenza vaccines for the US.
List of the 1 NDC products with the active ingredient Influenza A Virus Avictoria25702019 Ivr-215 h1n1 Antigen uv Formaldehyde Inactivated. Influenza A H1N1 2009 Monovalent Vaccine is formulated to contain 15 mcg hemagglutinin HA of influenza ACalifornia072009 H1N1 v-like virus per 05 mL dose. Kills Pandemic 2009 H1N1 influenza A virus formerly called swine flu Formula Size UPC.
Its Back - H1N1 Flu Returns in 2013-14. Ad Influenza Vaccine Live Intranasal.

2021 2022 Flu Shot Ingredients What Is In The Flu Shot And Why Fatherly

2021 2022 Flu Shot Ingredients What Is In The Flu Shot And Why Fatherly
Swine Flu Risk Factors Causes Symptoms
2

2021 2022 Flu Shot Ingredients What Is In The Flu Shot And Why Fatherly
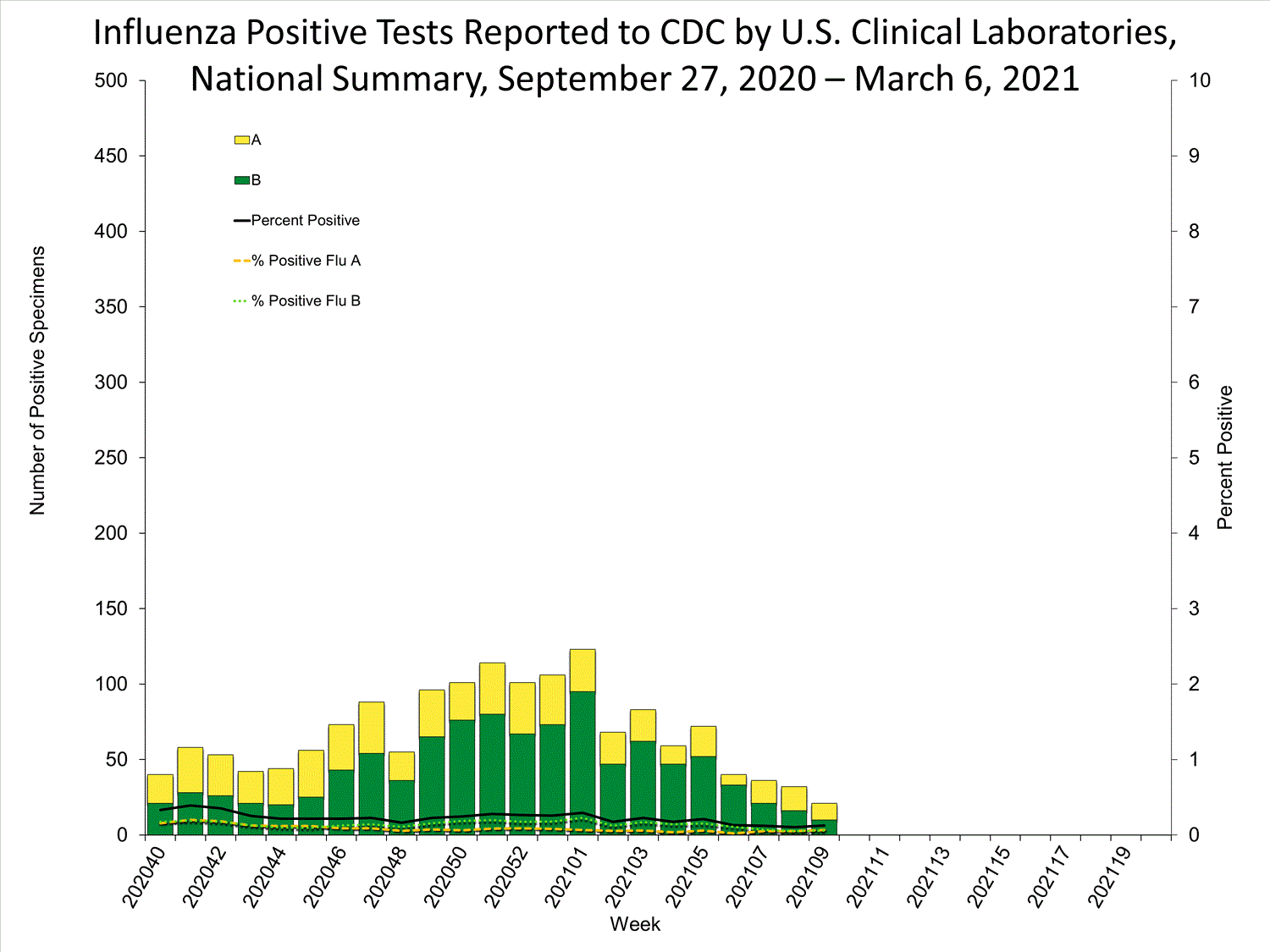
Fluview Summary Ending On March 6 2021 Cdc

Swine Flu Risk Factors Causes Symptoms
2

Genesis And Pathogenesis Of The 1918 Pandemic H1n1 Influenza A Virus Pnas

Genesis And Pathogenesis Of The 1918 Pandemic H1n1 Influenza A Virus Pnas

Fluad Quad Nps Medicinewise

Recommended Composition Of Influenza Virus Vaccines For Use In The 2022 Southern Hemisphere Influenza Season

Misinformation On Us Flu Shot Ingredients Resurfaces During Pandemic Fact Check

Genesis And Pathogenesis Of The 1918 Pandemic H1n1 Influenza A Virus Pnas

Hong Kong Influenza An Overview Sciencedirect Topics